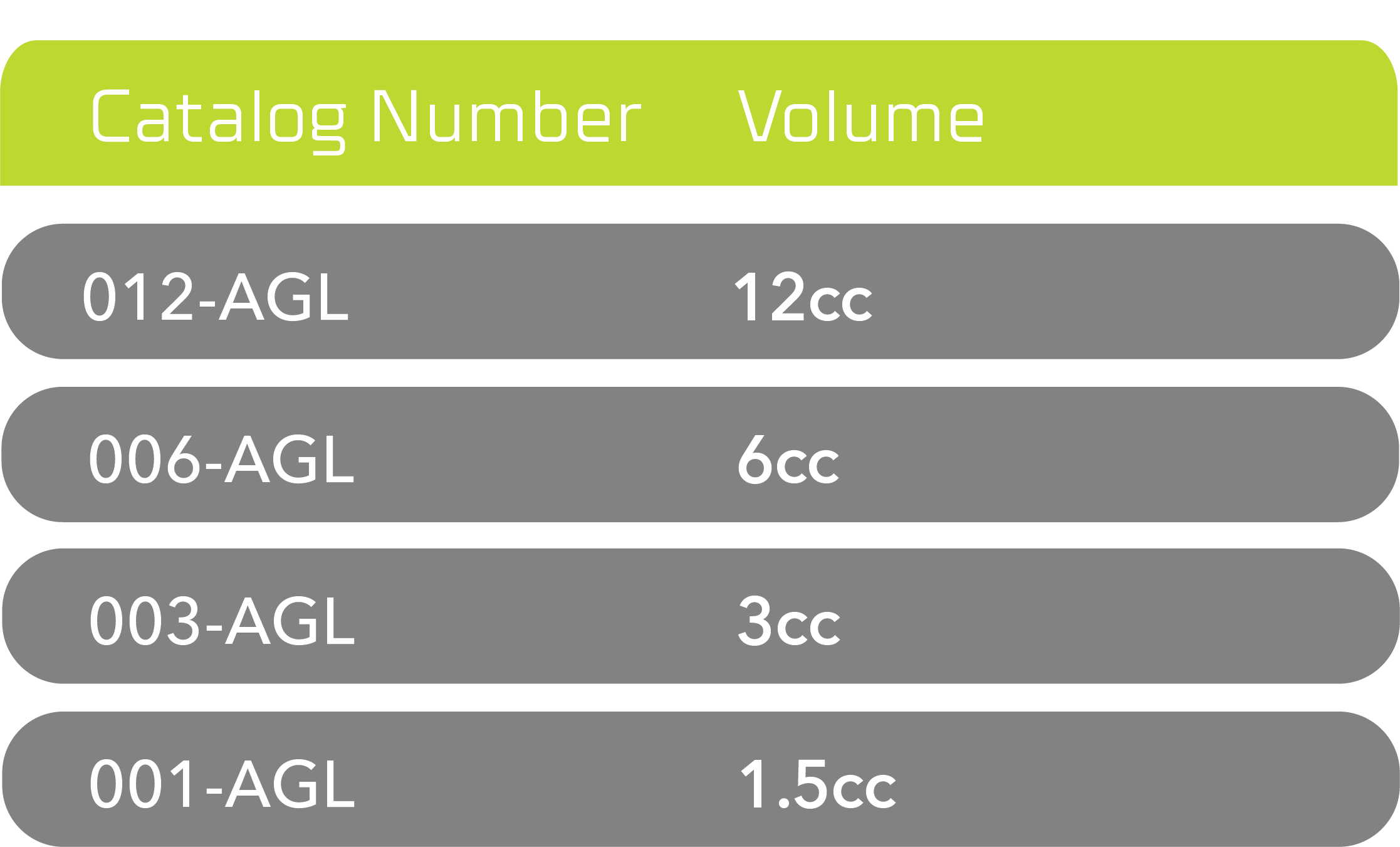
Agilon® Moldable
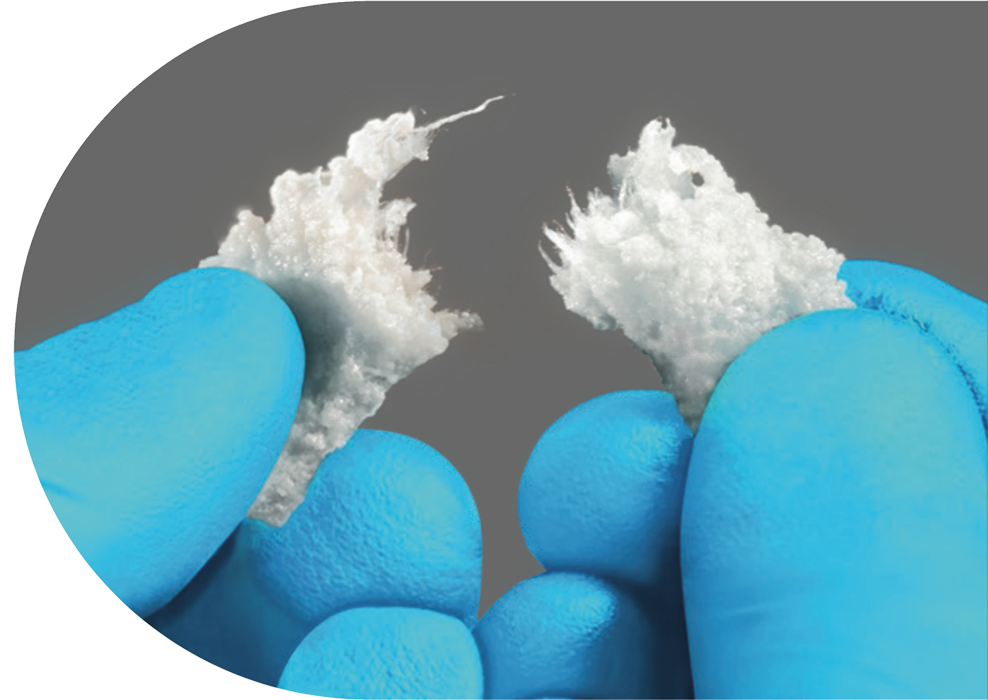
Agilon Moldable bone grafting product is the latest moldable grafting solution in Biogennix’ advanced bone graft product line. Developed with Biogennix proprietary TrelCor® technology, Agilon Moldable consists of a suspension of 1-2mm TrelCor granules in a rapidly absorbing, organic binder blended with type-1 collagen.
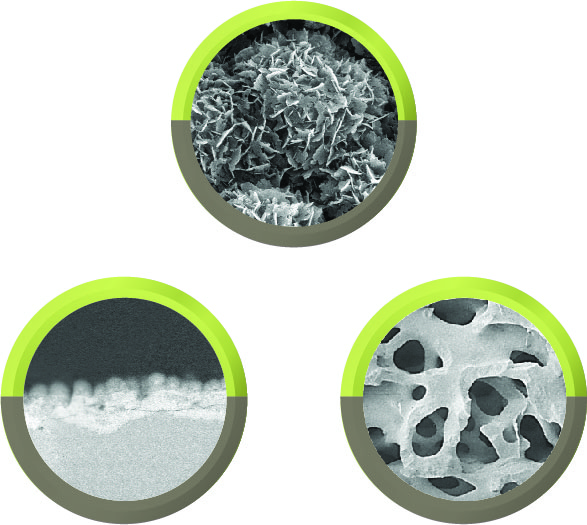
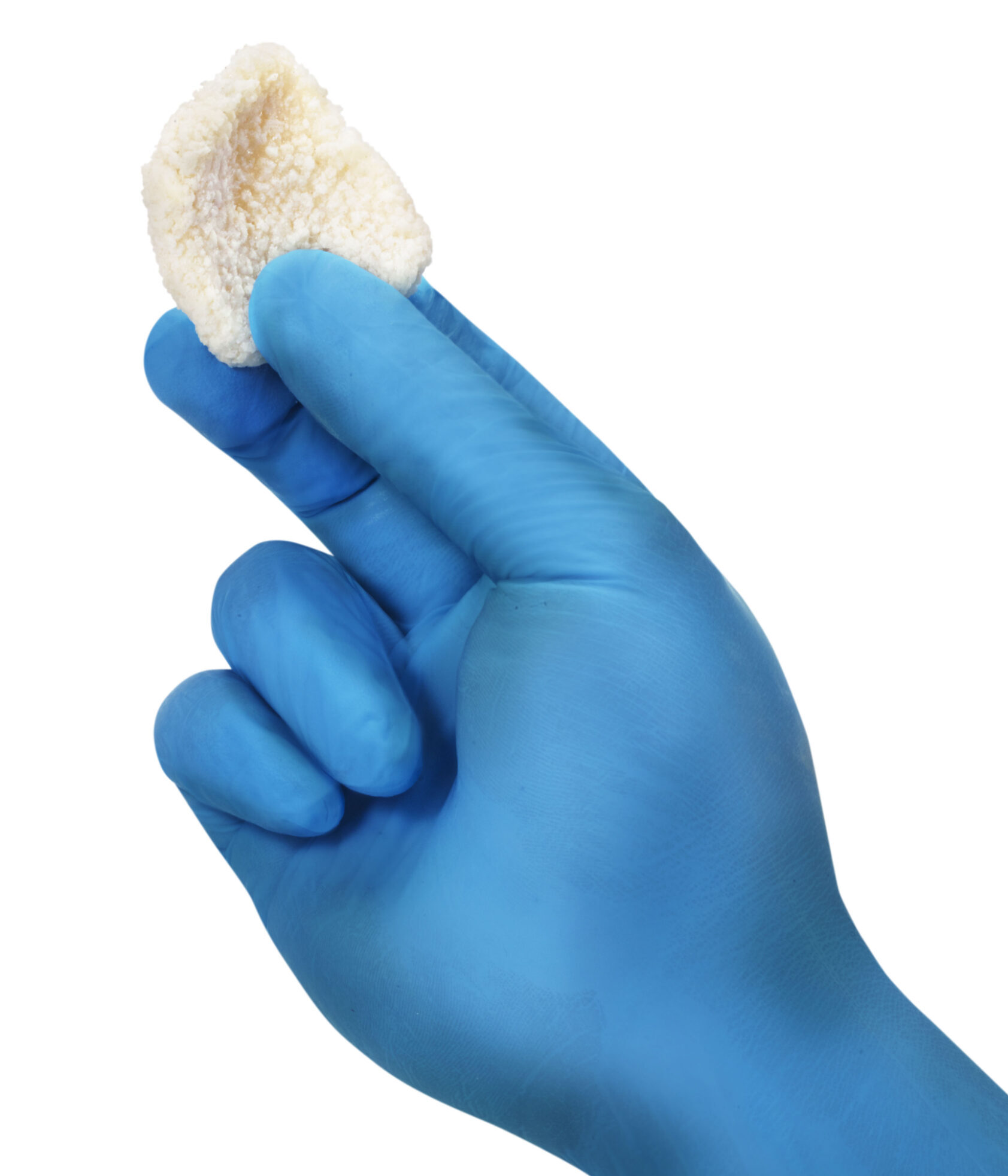
TrelCor granules provide enhanced bone formation due to the combination of a nanocrystalline surface with an HCA composition and interconnected pore structure that emulate human cancellous bone. The added collagen fibers in Agilon Moldable increase the overall cohesiveness, providing a firmer and more cohesive feel without sacrificing moldability. Additionally, the added collagen minimizes residual material left on surgical gloves, aiding in optimal handling characteristics during intraoperative placement. The organic binder resists breakdown during handling and placement, then is rapidly absorbed, leaving behind the porous TrelCor granules, which are resorbed, remodeled, and replaced with bone over the next 6-12 months.