Clinical Data
Biogennix advanced bone grafting products have been successfully implanted over 50,000 times in patients. Based on the innovative TrelCor technology platform, Biogennix bone grafting products provide surgeons with next-generation bone graft material that enhances the bone healing process.
These products are available in a variety of configurations that are compatible with commonly bone grafting techniques. All products are simple to use, can reduce OR time, and will provide unmatched intraoperative handling.
Common Surgical Uses
Spine
Foot & Ankle
General Orthopedics
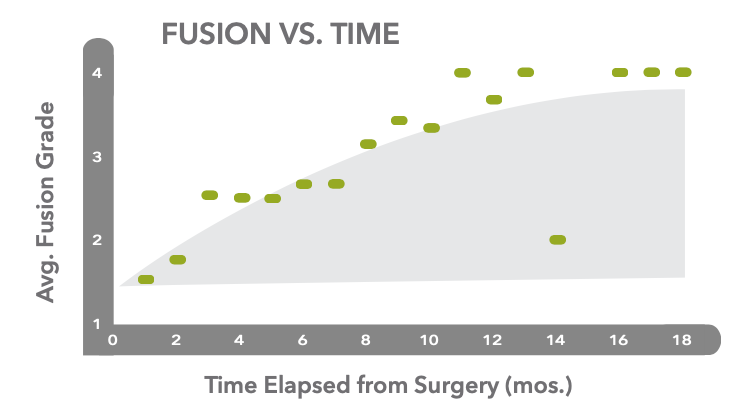
Spine Fusion Clinical Study
The clinical effectiveness of TrelCor advanced bone graft material granules was evaluated in a multi-center, 60-patient spinal fusion study. Patients were surgically treated with a 1-3 level lumbar interbody fusion. A combination of granules and autograft was used in the posterolateral space with standard interbody and posterior hardware placement. Radiographic fusion was assessed at each follow-up point using a 4 point scale: Grade 1 (no fusion), Grade 2 (incomplete fusion), Grade 3 (complete fusion), Grade 4 (solid fusion).
The results showed increasing fusion scores from 6 months to 1 year. At <6 months, the average fusion score was Grade 2 indicating a progressing fusion. At 7-10 months, scores increased to 3.2 with bridging fusion across the site. At 12 months, the fusion masses showed solid fusion with radiographically mature bone. The overall 12-month result showed fusion in 59 out of 60 patients (98% fusion rate). Overall both surgeons and patients were satisfied with the clinical outcomes.