Dual-Stage Bone Graft Resorption: The Unique Benefits of TrelCor Bone Graft Technology
Bone Graft Resorption: An Introduction
As covered in a previous blog about bone resorption, the processes of bone modeling and bone remodeling can be described as a coordinated interaction between stem cells, osteocytes, osteoblasts, and osteoclasts. Whether bone is modeling or remodeling depends on the balance between the resorption of existing bone and the formation of new bone.
The same cells involved in these processes also play a role in the healing response to bone graft materials. After a bone graft material is implanted in a patient during surgery, the bone formation response begins with new bone forming on the graft surface, and then through its porosity, if present. As more bone fills the implant area, the cells will initiate the graft resorption process. Ultimately, the goal of the implanted graft material is to fully fill in with new bone and be completely resorbed over time.
Regardless of the type of bone graft material implanted in a patient, the rate at which that graft resorbs plays an important role in the healing process. Most grafts resorb at a fixed rate, determined by their material composition. Unlike other synthetics on the market, Biogennix’s advanced TrelCor®-based graft materials provide optimal dual-stage resorption that matches the patient’s unique bone formation rate. This blog will describe the process of TrelCor resorption and its beneficial effects on the healing process in detail.
How to Tell that Bone Graft is Resorbing Over Time
Before we learn how TrelCor’s dual-stage resorption occurs, let’s first cover what resorption looks like radiographically. After any bone graft product is surgically implanted in a patient, clinicians may want to assess graft resorption to know how well the patient is healing after surgery. Typically, surgeons will use radiographs (X-rays) or computerized tomograms (CT’s) to see what is happening inside the patient over time. Depending on the composition of a bone graft material, its visibility on X-rays/CT’s can range from being radiolucent (not visible) to radiopaque (visible). During surgery and at patient follow-up appointments, there is value in having a relatively radiopaque graft material that can be visually identified. This allows clinicians to easily see the exact site where the graft material was placed and track bone forming in this area over time.
Some graft materials, like TrelCor, are visible on a radiograph. Due to this property, clinicians can visualize the progression of bone formation over time. Immediately following implantation, TrelCor-based graft materials have a granulated radiographic appearance with distinct edges. As new bone begins to form on the surface and within the porosity of TrelCor, the edges of the implant become less defined. Then, as resorption of the graft material occurs and the amount of new bone increases, the graft is no longer visible, and the site appears fully filled in with the patient’s own bone.
Controlling Resorption Through Material Composition
With standard ceramic synthetic bone graft materials, the composition of the graft material dictates whether the graft will resorb at a fast or a slow rate. For example, hydroxyapatite (HA) [Ca10(PO4)6(OH)2] and tricalcium phosphate (TCP) [Ca3(PO4)2] are both calcium phosphate ceramics commonly used as bone graft materials. Their different compositions result in a slow resorption rate for HA (~1% per year) and a faster resorption rate for TCP (6-24 months). These resorption times are inherent to the materials and can only be changed by combining HA and TCP together (called biphasic calcium phosphate – BCP) or adjusting the bone graft structure which impacts the surface area for bone growth and resorption.
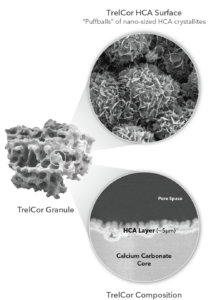
TrelCor is different from standard HA or TCP bone grafts in that it has a dual-region composition: the surface is comprised of hydroxycarbanoapatite (HCA) [Ca10(PO4)x(CO3)y(OH)z] and the core is calcium carbonate (CaCO3) (Figure 1). Since HCA resorbs more slowly compared to calcium carbonate, TrelCor resorption is directly controlled by the thickness of the slower resorbing HCA surface. As a result, the thickness of the HCA layer was optimized to ~5µm, which provides enough time for bone growth to occur over the surface before it is fully resorbed. This combination of HCA and calcium carbonate composition allows TrelCor to provide a patient-specific response.
How Dual-Stage Resorption of TrelCor Occurs
Patients resorb and remodel bone at different rates based on their age, health, the size of the bone defect, and other factors. The ideal resorption profile is one that can be controlled by each patient’s own bone formation rate. For example, small, contained defects in children will likely regenerate bone faster, so a faster resorbing graft would be ideal. Conversely, bone formation in an elderly patient undergoing a large, complex spinal fusion would occur at a slower rate. In this case, an optimal graft would last longer and have a slower resorption rate to match the slower bone formation rate of the patient.
The objective of a resorbable bone graft product is to function as a scaffold for as long as it takes to form new bone. Only then should the scaffold begin to resorb. With this in mind, TrelCor was specifically designed to resorb as bone grows into the porosity and onto the surface. As covered in Blog 19, osteoblasts (bone forming cells) signal the formation of osteoclasts (bone and graft resorbing cells), and vice versa. The below process and Figure 2 describe in detail how the dual-stage resorption of TrelCor-based graft materials occurs:
- Following implantation, bone formation initially occurs on TrelCor material directly in contact with the surrounding bone.
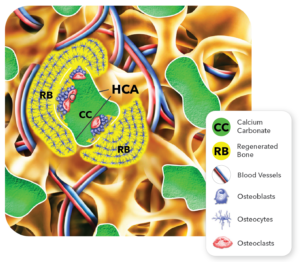
Figure 2: Key events that occur during bone regeneration and resorption of TrelCor bone graft products. - Bone growth gradually progresses over the surface of the TrelCor pore system until the entire granule is covered by new bone. This process continues to each adjacent granule.
- As the bone becomes thicker on the surface of the TrelCor granules, the osteoblasts in this new bone area signal the osteoclasts to begin the resorption process.
- Once activated, the osteoclasts begin resorbing the HCA surface layer. Since the HCA surface resorbs at a slower rate, this gives the bone formation response enough time to completely cover the TrelCor surface and fill in the porosity.
- After the osteoclasts have breached the HCA surface, they start aggressively resorbing the underlying calcium carbonate.
- New bone formation then enters the calcium carbonate area.
- Eventually, osteoclasts resorb the entire graft, and the process is complete. The area once occupied by the TrelCor graft has now been completely replaced with bone.
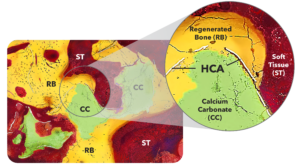
This effect can be seen clearly in histology images from in vivo studies that demonstrate the healing response of TrelCor materials. In one study, TrelCor was combined in a 1:1 ratio with autograft and implanted in the posterolateral spine of rabbits. At 12 weeks both a prominent bone formation response was visible, as was the resorption response. This timepoint specifically captured the initial resorption of the HCA layer and penetration of new bone into the calcium carbonate region. Figure 3 shows a SEM histology image that was colored to emphasize this effect. In this image, the dual composition of TrelCor granule is apparent with the calcium carbonate (green) and the HCA surface (white). Bone (yellow) is also seen growing on the surface of the granules and penetrating the calcium carbonate area.
Resorption of bone graft materials is important since bone grafts primarily function as a scaffold for new bone growth. If the scaffold resorbs too quickly (before bone formation is complete), then a void can develop, and the surgery can fail. If it resorbs too slowly, then the implant occupies space that should be filled in with new bone.
As seen from the TrelCor resorption process, the balance between bone formation and resorption can be controlled by the thickness of the HCA region. With decades of experience, Biogennix scientists have fine-tuned the HCA layer thickness to create an advanced bone graft material with an optimal resorption profile that is matched to each patient, i.e., “patient-controlled” resorption. This advanced bone graft property is unique to TrelCor with no other bone grafting product on the market possessing this ability.
In TrelCor’s dual-stage resorption response, if a patient forms bone quickly, for example in a young healthy patient, then more osteoclasts eventually become activated. This accelerates resorption of the TrelCor HCA layer and allows additional bone formation in the calcium carbonate area. Conversely, if the patient forms bone at a slower rate, for example in an elderly diabetic patient, then osteoclast activation also slows down. This gives the bone formation response enough time to progress at its own rate and eventually fill in the porosity of TrelCor. In this slower healing process, once enough bone is formed, the osteoclast process fully kicks-in and allows further bone formation in the calcium carbonate region.
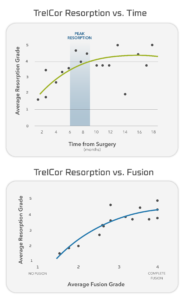
This “patient-controlled” resorption was seen in a 60-patient multi-center clinical study that evaluated the fusion and resorption response of TrelCor bone graft material implanted with autograft in spinal fusion patients. In this study, both fusion and resorption were radiographically assessed and scored on a scale of 1-4 (1=no fusion/resorption; 4=complete fusion/resorption). As shown in Figure 4, the progression of fusions is directly related to the progression of resorption with a peak resorption generally occurring by 6-9 months. This study confirmed the effectiveness of the dual-stage resorption of TrelCor.
While most commercially available bone graft products resorb at a fixed rate based on their material composition, next-generation advanced bone grafts were engineered to do more. Advanced synthetics like TrelCor-based bone graft products offer unique properties that benefit surgeons, hospitals, and especially patients. TrelCor bone grafts with their dual-phase composition allow for optimal “patient-controlled” resorption, providing significant advantages over “one-size-fits-all” first-generation materials still used today. It is no surprise that the market is increasingly seeing a shift towards more advanced synthetic bone graft materials, which offer distinct benefits over first-generation technologies.
As a recognized leader in advanced bone graft technologies, Biogennix is committed to bringing high-quality educational content to our field. This blog will cover technical topics ranging from basic bone graft science to advanced osteobiologic principles. We’ll also discuss market trends and industry challenges. We thank you for reading and invite you to learn more about us here.