Comparing the Cellular Effects of Bone Graft Materials: Nanosurfaces vs. Bioglass
One of the critical functions of a bone graft material is to support bone formation on its surface. This is the main reason why bone graft products contain osteoconductive surfaces. However, the level of biological activity occurring at the surface can vary depending on material composition and surface morphology. Ultimately, this can dictate how fast or slow bone forms within the graft. In bone graft surgical applications, many surgeons prefer a faster and more robust bone formation response since it directly impacts patient healing time and recovery. Bone graft materials that are actively involved in the bone formation process are advantageous since they can positively influence cells involved in bone healing. In the synthetic bone graft category, biologically active materials such as Bioglass or bioceramics with a nanosurface have the ability to enhance the cellular healing response. This blog will focus on the cellular effects of bone graft materials and how they can vary.
Bioglass Background
Bioglass (also called 45S5 bioactive glass) is a first generation, biocompatible, and resorbable material that was discovered in the 1960’s by Larry Hench, Ph.D. (Hench 1971). The specific 45S5 Bioglass composition is based on silica but also contains significant amounts of calcium, sodium, and phosphorus oxides: 45 wt% SiO2, 24.5 wt% CaO, 24.5 wt% Na2O, and 6.0 wt% P2O5. The inclusion of these elements into the silica glass makes the material biocompatible and allows it to be slowly dissolved in the body over a 6-9 month period.
Once Bioglass is implanted, the water in interstitial body fluids begins to slowly dissolve the material. During this process, water soluble dissolution ions containing silica, calcium, phosphorus and sodium are released to the local environment. Early in the discovery of Bioglass, it was found that these dissolution ions can combine with ions in body fluids to form a layer of calcium phosphate mineral on the surface of the glass. This property is called bioactivity and results in direct bonding of the Bioglass surface to the newly formed bone (Hench 1973). Interestingly, it wasn’t until the late 1990’s/early 2000’s that testing showed that the dissolution ions responsible for bioactivity also had a positive influence on cells (Wilson 2000).
Stimulating Cellular Effects of Bioglass (Osteostimulation)
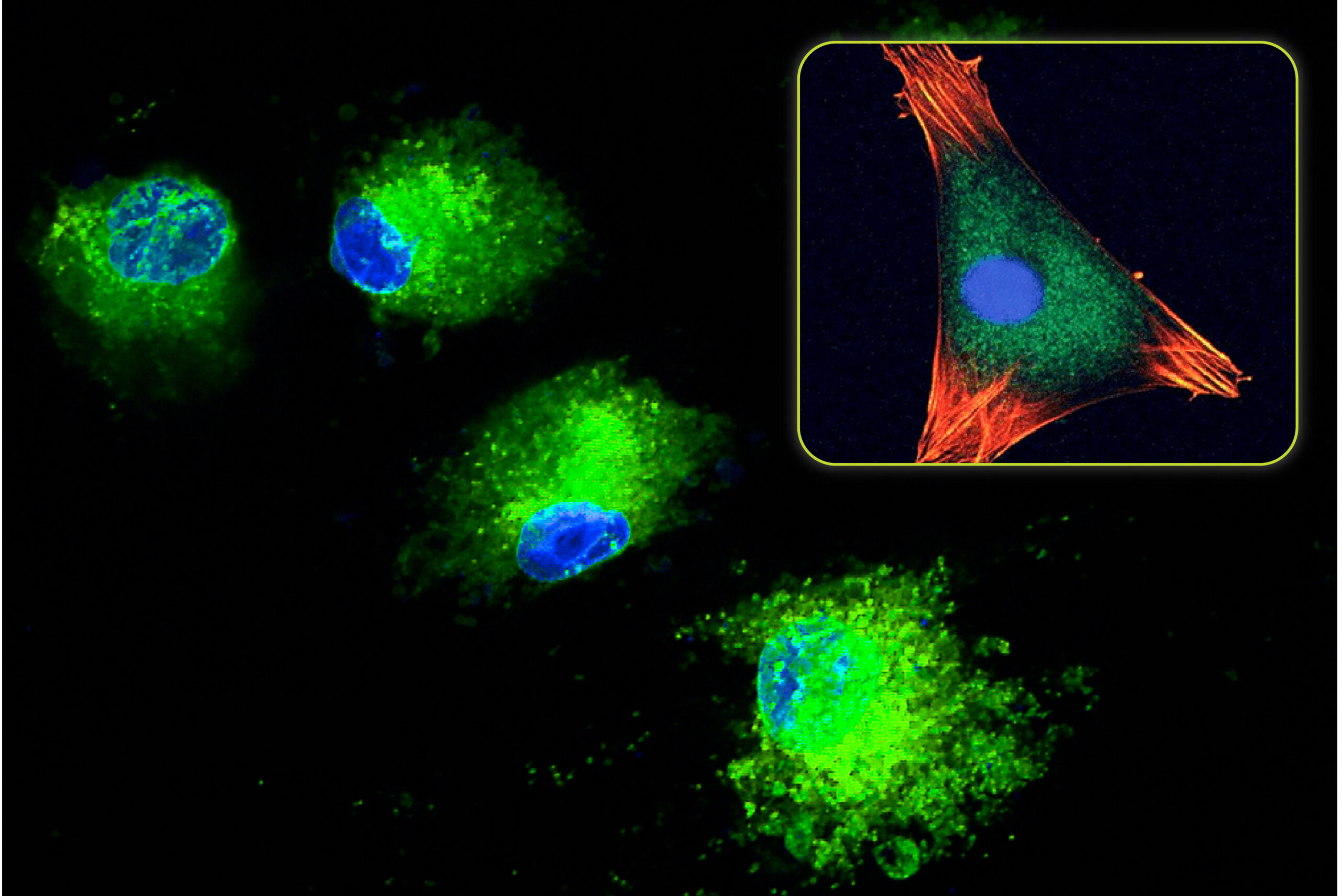
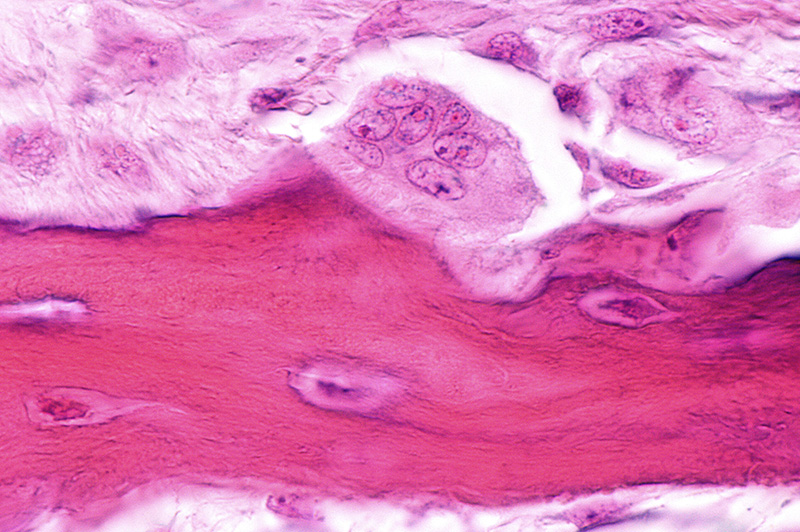
The bone formation response is primarily dependent on osteoblasts and stem cells that are found at the bone graft site. As discussed above, Bioglass releases ions as it slowly dissolves over time. Eventually these ions build up near the Bioglass surface and a bioactive layer is formed. As the cellular bone formation response is initiated, cells at the surface of the Bioglass bone graft are exposed to the ionic dissolution products. Studies have shown that the exposure of osteoblasts to Bioglass dissolution ions increases cell proliferation and cell function (Xynos 2000). It was also shown that dissolution ions could differentiate stem cells into osteoblasts (Bosetti 2009). The ability of Bioglass to interact with cells is called osteostimulation and is recognized as its main bone healing property (Hu 2009).
Due to the osteostimulation mechanism of action and its direct link to ion release, the healing process is dependent on the dissolution and build-up of ions in the graft area and the formation of the bioactive layer. Since this process takes time, bone formation on the Bioglass surface can’t start until these processes are completed.
Stimulating Cellular Effects of Nanosurfaces
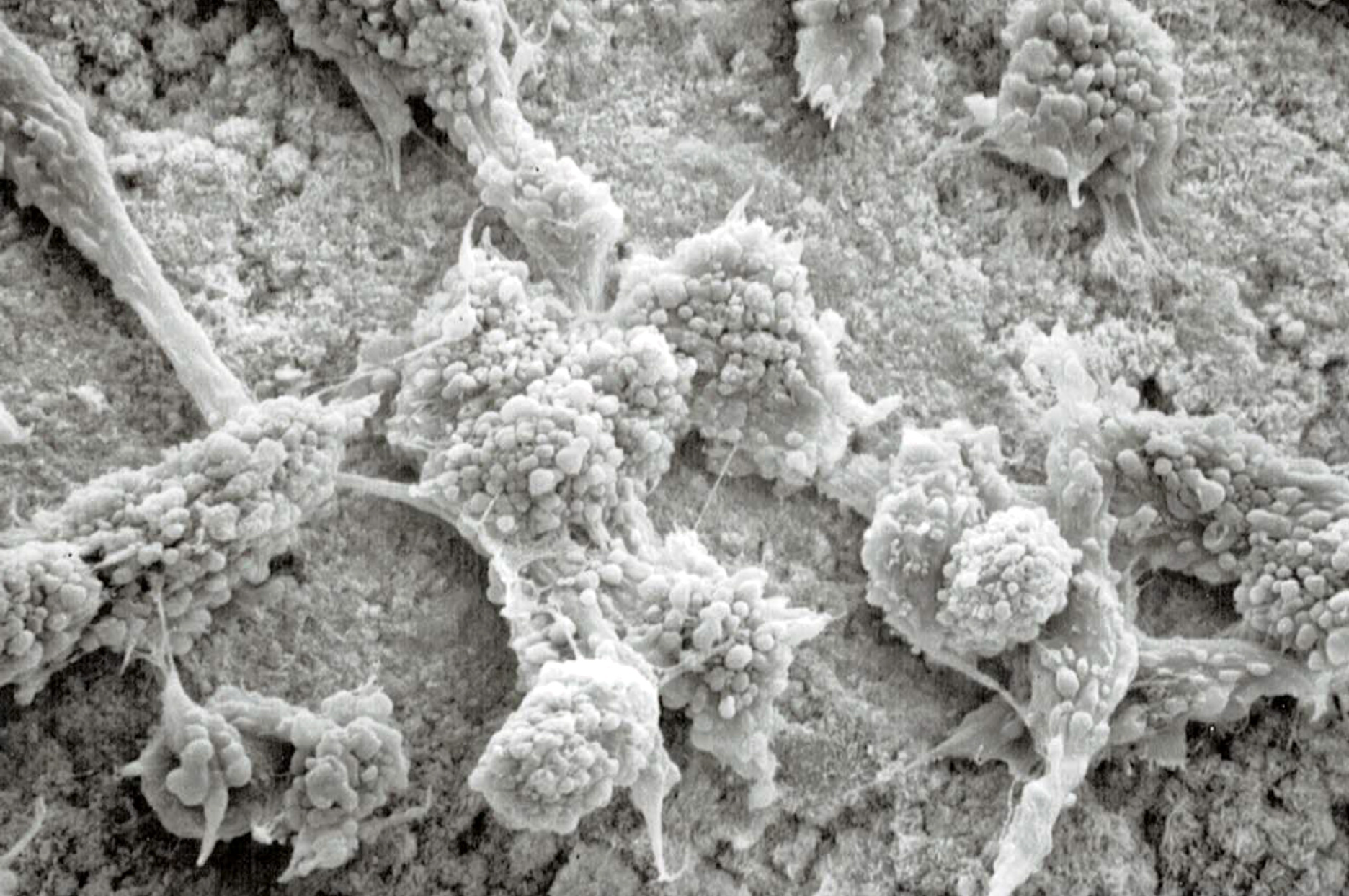
Bioglass represents one of the first synthetic bone graft materials that can directly affect the cellular bone formation process. Recently, advancements in surface technology have introduced a new class of innovative bone graft materials that also result in positive cellular effects. Similar to Bioglass, studies have shown that nanostructured surfaces can increase osteoblast proliferation and function, and cause stem cell differentiation.
Although the end result is the same, the mechanism on how this occurs is different. The nanosurface provides direct mechanical stimulation to the cell membrane when osteoblasts and stem cells directly attach to the surface. Comparatively, an osteoblast is approximately 10,000 nm (10 µm) wide while most nanosized surface features are <100 nm. When the cell attaches to this type of surface, it comes in contact with thousands of nanosized surface features that interact with the cell membrane. This mechanically strains the cell membrane which activates bone’s inherent ability to form more bone in response to stress (known as Wolff’s law).
The nanosurface also allows for localized dissolution and ion release from bioceramic material, and protein adsorption due to the extremely high surface area created by the nanofeatures. The combination of these properties creates a surface that can positively interact with cells and influence bone formation process.
Additionally, nanostructured bone graft materials are manufactured with a cell-accessible surface ready to go. In contrast to Bioglass, a cell-stimulating nanosurface is immediately available to local cells, without requiring time to partially dissolve, form a bioactive layer, and build up enough dissolution ions to affect the local cells. This allows the cellular bone formation process on a nanosurface bone graft to start as soon as the material is implanted.
Bioceramics Are a Viable Alternative To Bioglass
Bioglass and bioceramics with nanosurfaces are biologically active bone graft materials that influence various cellular effects through different mechanisms of action. While Bioglass products require time to form a bioactive layer, nanostructured bone graft materials, like Biogennix’s TrelCor® technology, contain a cell-accessible surface that works immediately upon implantation. For clinical practices that want to use the most cutting-edge bone graft materials, Biogennix advanced synthetic products are excellent surgical options.
As a recognized leader in advanced bone graft technologies, Biogennix is committed to bringing high-quality educational content to our field. This blog will cover technical topics ranging from basic bone graft science to advanced osteobiologic principles. We’ll also discuss market trends and industry challenges. We thank you for reading and invite you to learn more about us here.