The Power of High Granule Density in Bone Formation Response

Improve the bone formation response by taking a critical look at the function, granule density, and porosity of the synthetic bone products you choose.
The main function of a synthetic bone graft material is to support bone formation around the graft particles and through the porosity. However, this process does not spontaneously occur within the graft. For optimal bone formation, a synthetic bone graft material must make direct contact with the surrounding bone at the implant site. It is this surface where the cellular bone formation process is initiated. As healing progresses, bone grows from the graft interface and eventually forms throughout the graft material. This bone in-growth process plays an important role in full healing of the graft site and can be directly affected by the granule density and form of a bone graft product.
The Challenge of Bone Graft Granule Density vs. Handling
 Over the years, synthetic bone graft products have evolved to provide surgeons with better intraoperative handling and graft containment. Some of the earliest synthetic bone graft products developed were loose granules (pictured to the left). In a loose granule form, surgeons were ensured that they had a high content of graft material. However, loose granules on their own can be difficult to implant and tend to migrate from the site.
Over the years, synthetic bone graft products have evolved to provide surgeons with better intraoperative handling and graft containment. Some of the earliest synthetic bone graft products developed were loose granules (pictured to the left). In a loose granule form, surgeons were ensured that they had a high content of graft material. However, loose granules on their own can be difficult to implant and tend to migrate from the site.
Handling and granule containment were improved with the introduction of moldable and flexible synthetic bone graft products that combined the individual granules with a carrier. This resulted in putty and sheet products that significantly improved the interoperative handling and ease of use of these products.
Although the interoperative handling improved with these new bone graft forms, the introduction of a carrier to many of the bone graft products on the market resulted in a lower bone graft granule density. Since bone graft granules are the sole component responsible for osteoconductive bone formation, the bone graft granule density plays an important role in achieving good clinical results.
Surgical Preparation for a Proper Bone Formation Response
In most surgical applications, a synthetic bone graft material is not just simply implanted at the graft site. The area is first prepared by the surgeon to facilitate a proper bone formation response. This is typically accomplished by modifying the bone graft surface using burrs, drills, and/or saws to expose a uniform surface free from soft tissue and initiate a healing response.
In the case of fusion surgery at a motion segment (e.g., foot, ankle, and spine fusion), the graft site preparation plays a critical role in obtaining an optimal bone formation response. Fusion surgery is typically performed to treat arthritic joints in the foot, ankle, and hand, or at a degenerative intervertebral space in the spine. At these joints, the bone is covered by a layer of cartilage. Placement of bone graft material on a cartilage surface will not result in a new bone formation response, and a non-union will occur.
Therefore, it is particularly important in fusion procedures to fully remove the cartilage and outermost layer of cortical bone (decortication). This process exposes underlying cancellous bone and marrow (called “bleeding bone”). Although this technically “damages” the bone, it also initiates a cellular healing response that is beneficial for the bone formation response.
Bone Formation Response Process at a Graft Site
Once the bone graft site is properly prepared, a synthetic bone graft product can be placed. The material is delivered to the site and pressed in place to maximize direct contact with the “bleeding bone” surface. Instruments such a tamps can also be used to pack down the bone graft material and ensure maximal contact with surrounding bone. With the bone graft material directly in contact with the “bleeding bone” surface, it becomes involved in the healing process.
The bone formation response is summarized in the following sequence:
- Initiation of Cellular Bone Healing: Due to the exposure of “bleeding bone” during the graft site preparation and decortication processes, a cellular bone healing response is initiated.
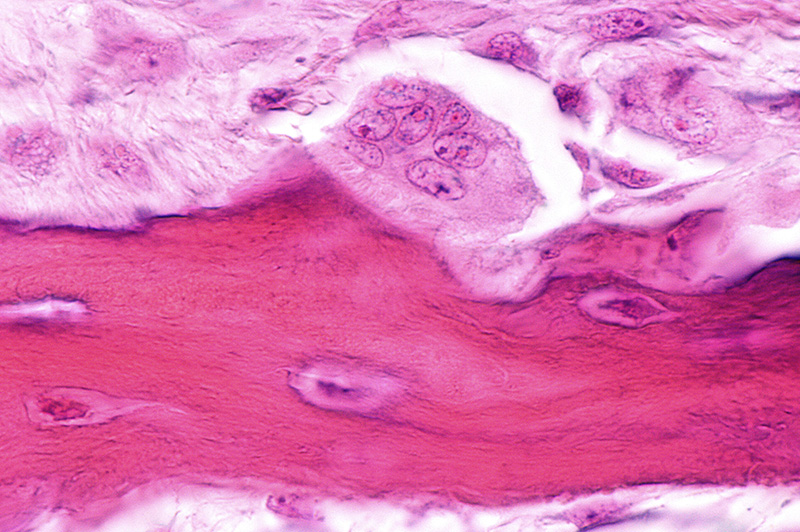
- Interface Bone Formation: Cells at the bone-graft material interface begin forming bone. This extends from the existing bone and into the bone graft material at the interface.
- Bone Formation on the Graft Material: Cells migrate into the porous bone graft material and attach to the surface. Bone formation that was initiated at the interface begins to advance onto the bone graft material in contact with the surrounding bone.
- Bone In-growth Process: The bone formation that started at the interface continues throughout the porosity of the granules that are in contact with the surrounding bone. Once this is complete, the bone formation front continues deeper into the graft area and spreads to other granules at the site. Eventually bone formation will completely span the entire graft area.
- Graft Resorption/Complete Healing: As the bone formation process continues throughout the entire graft area, the graft material is also resorbing. This creates new space which eventually fills in with additional bone. Once the graft is fully resorbed, the area is completely filled in with new bone.
The bone in-growth process occurs at all areas where the bone graft materials are directly contacting the surrounding bone. As a result, synthetic bone graft products with higher granule density will have more contact with the surrounding bone and can elicit a stronger osteoconductive bone formation response.
A Closer Look at the Role of High Granule Density
As stated before, bone graft product handling can be improved using resorbable carriers. This allows for various moldable and flexible bone graft forms to be created, such as putties and strips. The main function of these carriers is to improve interoperative handling and ease of use.
For example, synthetic bone graft putties are created by combining granules or particulate material with sticky or waxy carrier. As more granules are added, the stickiness is reduced. The result is a doughy, pliable, and cohesive material. In bone graft sheet products, collagen is typically used as a carrier to create a suspension of granules within a freeze-dried collagen fiber scaffold. When hydrated, these sheets become flexible and pliable.
In both putties and sheets, the addition of the carrier significantly improves handling and granule containment. However, in many products, as more carrier is added and handling improves, the bone graft granule density is reduced. Since the healing process at a bone graft site is highly dependent on the direct contact of the bone graft granules with the surrounding bone, a reduced granule density can limit the bone formation response.
The improvement to intraoperative handling of a synthetic bone graft must be balanced with maintaining a high granule density. Implantation of a bone graft product with excellent handling (but a low granule density) can give the surgeon a false sense that the graft area is filled. Following bone graft implantation, the bone graft site may appear visibly filled. However, once the carrier is resorbed, the remaining granules will settle, and the graft volume will be reduced. Without a high granule density, the remaining granules will only make partial contact with the surrounding bone. This can limit or delay the bone formation response.
Improve Bone Formation Response with High Granule Density AND Optimal Handling
Synthetic bone graft products can be developed with the dual goals of providing excellent handling and a high granule density. This is accomplished by carefully selecting a cohesive and moldable carrier that is compatible with the granule shape, size, and porosity.
Biogennix has utilized this approach in the development of all of its advanced synthetic bone graft products, including its latest offerings Agilon® Moldable and Agilon Strip. Biogennix bone graft products all have excellent handling and a high granule density. The high granule density is due the cohesive carrier interacting with the biomimetic porosity within the granules. Due to the carrier choice and structure of the TrelCor® granules, a smaller amount of carrier can be used.
In the Biogennix products, this high granule density concept is similar to filling a bucket full of golf balls with water. As the carrier is added to a Biogennix product, the carrier fills the spaces around the granules and within the porosity (similar to water filling the space around the golf balls). When the carrier is fully added, the total granule density of the finished produce doesn’t significantly change. This results in a bone graft with granule content that is nearly 100% by volume, combined with the benefits of excellent intraoperative handling.
Due to the high granule density, Agilon Moldable and Agilon Strip advanced synthetic bone graft products will completely fill a bone graft site with granules, which maximizes direct contact with the surrounding bone even after the carrier is resorbed.
As a recognized leader in advanced bone graft technologies, Biogennix is committed to bringing high-quality educational content to our field. This blog will cover technical topics ranging from basic bone graft science to advanced osteobiologic principles. We’ll also discuss market trends and industry challenges. We thank you for reading and invite you to learn more about us here.