The Effect of Surface Composition on Bone Formation
Since their introduction to the market in the 1990s, synthetic bone graft materials have been continually improving and evolving. Over the years, bone graft scientists and engineers have discovered novel materials and manufacturing techniques that can enhance bone formation and improve patient outcomes. As a result of this work, several key characteristics known to impact bone formation have been discovered. This includes:
- Surface morphology
- Surface composition, also referred to as surface chemistry
- Porosity
- Mechanical strength
- Intraoperative handling
This article focuses on the importance of bone graft surface chemistry and the impact of surface composition on bone formation.
Background: Bone Mineral Composition
Bone is a composite material composed of collagen fibers and bone mineral, with small amounts of non-collagenous proteins (e.g., growth factors). Although textbooks often refer to the mineral content of bone as hydroxyapatite, an accurate description is more complex. The bone mineral component consists of carbonated apatite (also called hydroxycarbanoapatite) with minor elemental substitutions, including magnesium, iron, sodium, and zinc.
Bone is a dynamic tissue that is constantly subjected to a remodeling process (resorption and new bone formation). The bone mineral composition plays an active role in the new bone formation process by acting as a source of calcium and phosphate ions, as well as these minor elements. Solubility of calcium phosphates is controlled by many variables, including surface area and crystallinity, but is also a function of the ratio of calcium to phosphorus. The lower the calcium/phosphorus ratio, the higher its solubility.
Due to the importance of calcium and phosphate in the bone formation process, initial synthetic bone grafts introduced to the market were based on calcium phosphate ceramics. These materials were found to be effective bone graft scaffolds that could support bone formation directly on the material surface.
Types of Calcium Phosphate Ceramics Used as Synthetic Bone Grafts
Calcium phosphate ceramics are commonly used as porous bone graft scaffolds. Although these materials contain similar calcium and phosphate components, differences in composition (i.e., the calcium-phosphate ratio) result in varying properties. Common calcium phosphate bone graft materials include the following:
- Hydroxyapatite (HA): HA was one of the first synthetic bone graft materials on the market. It is composed of the major calcium and phosphate elements of bone mineral without any of the substituted elements. Moreover, HA implants are manufactured by sintering which causes the formation of large crystallites and low surface area. This decreases its solubility in aqueous fluids (e.g., body fluid) as well as by osteoclasts. This results in an extremely long resorption process (~<1% resorption per year) which makes sintered HA functionally non-resorbable. The slow surface dissolution prevents pure HA implants from being bioactive.
Chemical Formula: Ca10(PO4)6(OH)2
- Tricalcium Phosphate (TCP): TCP was developed to address the slow resorption time of HA. The lower calcium/phosphorous ratio for TCP (3/2 or 1.50) increases its solubility and resorption compared to HA (10/6 or 1.66) (Hench 2013). This allows TCP to be resorbable through both solution and cell-mediated processes. TCP is typically fully resorbed in 9 months. Similar to HA, a cellular-surface preparation phase occurs prior to bone formation.
Chemical Formula: Ca3(PO4)2
- Biphasic Calcium Phosphate (BCP): BCP ceramics are a composite of HA and TCP. BCP was developed to allow the material properties of the components to be modified by altering the ratio of slowly resorbing/non-resorbable HA with faster resorbing TCP. Due to the combination of materials, BCP is resorbed by both solubilization and osteoclast resorption. As with HA and TCP, a cellular surface preparation phase is required prior to new bone formation.
Chemical Formula: chemical mixture of Ca10(PO4)6(OH)2and Ca3(PO4)2
- Hydroxycarbanoapatite (HCA): Compared to the other calcium phosphate ceramics, HCA is the material that most closely resembles bone mineral. The addition of carbonate to HA creates a resorbable ceramic that has a resorption profile similar to TCP (solution and cell-mediated processes). Like bone, the carbonate in HCA replaces the hydroxy (OH) group (Type A substitution) and the phosphate (PO4) group (Type B substitution) (Ren 2012). Due to the carbonate substitution, HCA results in a cellular healing response that is similar to natural bone. Most notably, the cellular preparation phase of the HCA surface composition is significantly shortened, leading to faster bone formation in the earliest phase of healing.
Chemical Formula: Ca10(PO4)x(CO3)y(OH)z
Cellular Response to Calcium Phosphate Ceramics
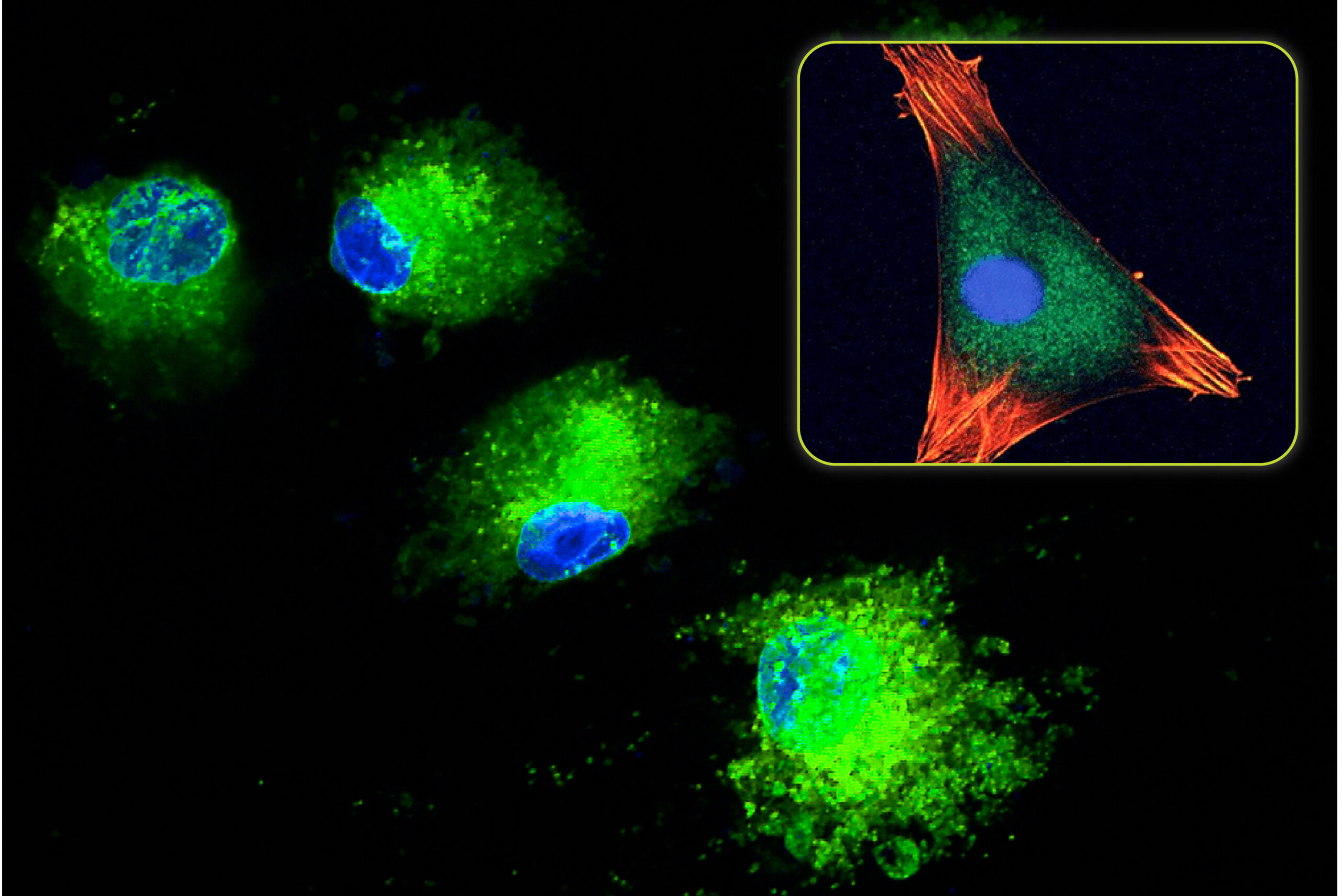
When standard calcium phosphate ceramics (HA, TCP, and BCP) are implanted, the initial cellular response involves macrophages and osteoclasts. These cells begin a conditioning process that prepares the ceramic for eventual bone formation (Spence 2006). This involves changes to the surface topography (through osteoclast resorption) and the deposition of signaling proteins. Once this process is concluded, the bone formation process is able to begin.
With HCA material, the cellular conditioning process appears to be shorter (Spence 2009). The osteoclast resorption process and subsequent change to the surface topography occur faster in HCA compared to other calcium phosphate ceramics. This is attributed to the increased solubility and faster ion release of HCA compared to these materials. These effects were studied in vivo by Hayashi who compared the bone formation response of identical scaffolds composed of HCA, TCP, and HA (Hayashi 2019). The result from this study showed that the HCA implant produced the highest amount of mature bone compared to TCP and HA. At 4-weeks, the average HCA mature bone area was 4.3X higher than TCP and 14.3X higher than HA. This represented a significant increase in the bone formation response.
Next-Generation Surface Composition for Bone Formation
A variety of calcium phosphate ceramics have been used to create synthetic bone graft materials. These ceramics have varying compositions, resorption times, and cellular responses once implanted. In general, calcium phosphate materials have been shown to be effective osteoconductive scaffolds. However, recent work examining the effect of surface composition on bone formation has shown that the bone formation rate can be influenced. Due to its similarity to naturally substituted bone mineral, the carbonated apatite in HCA results in a shortened cellular conditioning phase and a faster bone formation response.
In Biogennix’ TrelCor® advanced bone graft material, a calcium carbonate scaffold with biomimetic porosity is subjected to a chemical conversion process that converts the outer region of calcium carbonate to nanocrystalline HCA. This unique process allows for direct control over early ion release and subsequent matrix by controlling the thickness of the HCA layer. Biogennix has optimized this process to provide an advanced graft material that capitalizes on the established advantages of an HCA surface composition to positively influence bone formation.
As a recognized leader in advanced bone graft technologies, Biogennix is committed to bringing high-quality educational content to our field. This blog will cover technical topics ranging from basic bone graft science to advanced osteobiologic principles. We’ll also discuss market trends and industry challenges. We thank you for reading and invite you to learn more about us here.